GTHX - Trilaciclib CRC Phase 3 Data Coming!
- BPIQ

- Jan 25, 2023
- 4 min read
Summary:
GTHX Trilaciclib Phase 3 data in colorectal cancer is expected in February 2023
This is initial PRESERVE 1 CRC data and initial PFS data expected in Q4 2023
So far no CRC data from Trilaciclib has been reported - Ph2 data was from breast cancer patients
Get Amp's view/post of GTHX ahead of the mCRC data. If you are a BPIQ Pro member, contact support@BPIQ.com to for help adding an Amp subscription.
Trilaciclib
Trilacaclib is a small molecule cylin-dependent kinase (CDK) 4/6 inhibitor that is already approved to treat small cell lung cancer under the name COSELA. Tricaclib is currently in several late stage clinical trials for cancer including colorectal cancer, breast cancer, and bladder cancer. These trials are evaluating trilaciclib in combo with chemotherapy regimens, specifically FOLFOXIRI for the CRC trial.
The Phase 3 PRESERVE 1 trial is evaluating trilaciclib before treatment with FOLFOXIRI and bevacizumab for patients with metastatic colorectal cancer (mCRC). The primary myeloprotection endpoint for this trial is to evaluate severe neutropenia during induction and duration of severe neutropenia, Grade 3 or 4 diarrhea, and patient outcome data.
Currently the doublet therapy of FOLFOXIRI and bevacizumab is the best treatment for 1L CRC patients, but is very myelotoxic and only works for a small population, so the addition of Trilaciclib could work as a myeloprotective agent for these patients.
PRESERVE 1 Data
PRESERVE 1 data in CRC is expected in February!
This will be the initial results from the pivotal Phase 3 trial, and the first Trilaciclib data in CRC. Progression Free Survival (PFS) data is expected in Q4 2023. If data expected next month is positive, COSELA could launch in 2024 for CRC.
Patients in this trial include first line (1L) CRC patients, which includes MSS patients. This is the same type of colorectal cancer that AGEN's Botensilimab is treating. See our previous CRC/ASCO-GI article for more info on the AGEN data presented and what is to come for the company HERE.
The primary endpoint of the PRESERVE 1 trial is myeloprotection to include severe neutropenia during induction & duration of severe neutropenia during Cycles 1-4 of treatment.
Severe neutropenia is characterized as an abnormally low count of neutrophils ( white blood cells), specifically less than 500 / microliter. This often occurs with chemotherapy and can lead to shorter duration of the treatment.
Also being evaluated is the impact of trilaciclib on Grade 3 or 4 diarrhea: Grade 3 typically requires a hospital stay and Grade 4 can be life-threatening.
Currently, the cases of Grade 3 or 4 diarrhea in patients treated with FOLFOXIRI + bevacizumab is 17.8% and the cases of neutropenia is 45.8%. Patients treated with doublet chemotherapy + bevacizumab had Grade 3 or 4 diarrhea in 8.4% of patients and neutropenia in 21.5% of patients. The FOLFOXIRI treatment is more efficacious (ORR of 64.5% vs 53.6% in doublet + bevacizumab therapy) but also more myelotoxic, which is why adding trilaciclib to the regimen is being assessed. If trilaciclib can positively impact the occurrence of diarrhea and neutropenia, then there could be an improvement in tolerability of FOLFOXIRI treatment.
Secondary endpoints for this trial include PFS (progression free survival) (expected Q4 2023) and OS (overall survival) (also expected in Q4 2023).
The primary endpoint data in February could enable sNDA submission. If Trilaciclib data is positive, it could become the new standard of care for 1L CRC. See Figure 1 for more info on the upcoming readout.
Fig. 1 Key readouts coming from PRESERVE 1 trial

Previous Trilaciclib Data
Previous Phase 2 data for Trilaciclib was reported in December 2020 and was from the triple negative breast cancer trial. This trial evaluated Trilaciclib in combo w/ a chemotherapy regimen of gemcitabine/carboplatin (GC).
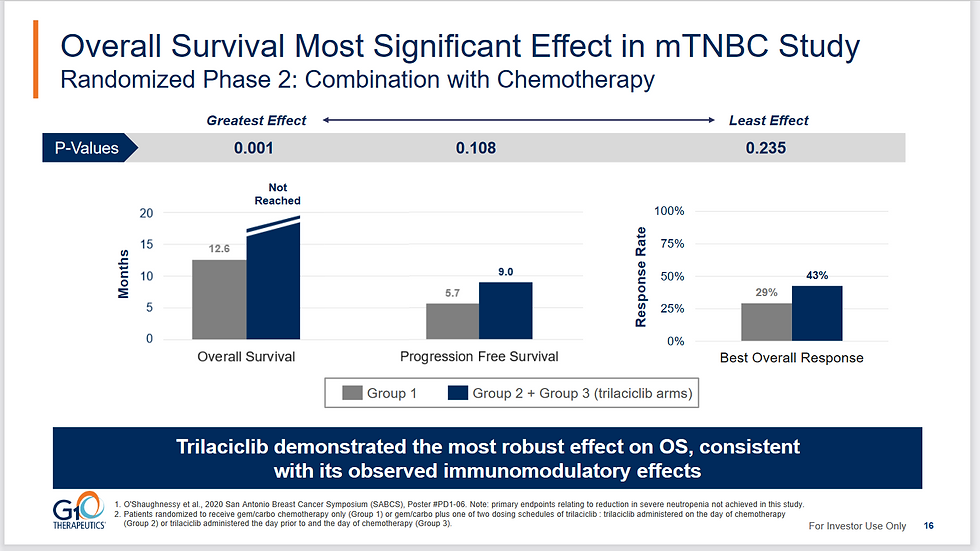
In the Phase 2 TNBC trial, Overall survival was 12.6 months in the GC alone group, OS was not reached in group 2 (trilaciclib) and OS was 17.8 months in Group 3 (also trilaciclib). The median Group 2 and 3 OS was 19.8 months. There was no previous Phase 2 CRC data, but after positive Ph2 breast cancer data they extended the asset to include trials in CRC. See Figure 2 for this Phase 2 data.
Interim OS data from Ph3 TNBC PRESERVE 2 data is expected in H2 2023.
Fig. 2
Other CDK4/6 and CRC assets
Trilaciclib is a CDK4/6 inhibitor; we scoured the BPIQ database to determine other assets treating cancer that are also CDK4/6 inhibitors.
ONTX Narazaciclib (CDK4/6 & ARK5 inhibitor) for solid tumors
NUVB NUV-422 (CDK inhibitor) for glioma, mBC, and mCRPC
TLSA Milciclib (pan-CDK inhibitor) for solid tumors and HCC
MEIP Voruciclib (CDK Inhibitor) for B-Cell malignancies and AML
ARVN ARV-471 (oral ER-targeting PROTAC® protein degrader) for mBC
Also see Table 1 for a list of other upcoming CRC readouts and events. After the positive AGEN data and approval of SGEN & MRK's TUKYSA last week, 2023 has already been an exciting year for CRC. We hope for even more impressive data in February and the rest of the year.
Table 1. Upcoming CRC events and readouts
This article is not investment, tax, or legal advice. Please do your own diligence and seek advice from professional advisors representing your interests.
Article history: 1/25/23 initially published post

Comments